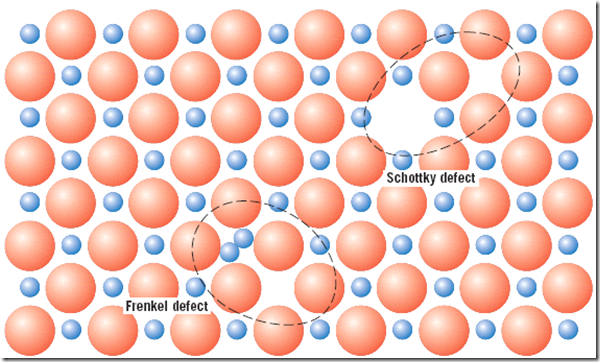
Frenkel defect: Defect involving a cation–vacancy and a cation–interstitial pair is called a Frenkel defect. It might be thought of as being formed by a cation leaving its normal position and moving into an interstitial site. There is no change in charge because the cation maintains the same positive charge as an interstitial. There is no change in charge because the cation maintains the same positive charge as an interstitial.
Schottky defect: A cation vacancy–anion vacancy pair known as a Schottky defect. This defect might be thought of as being created by removing one cation and one anion from the interior of the crystal and then placing them both at an external surface. Since both cations and anions have the same charge, and since for every anion vacancy there exists a cation vacancy, the charge neutrality of the crystal is maintained.
Burgers vector: http://en.wikipedia.org/wiki/Burgers_vector
Lattice constant: http://en.wikipedia.org/wiki/Lattice_constant